
The “ORS” Controversy: Why Authenticity Matters?
The Food Safety and Standards Authority of India (FSSAI) recently issued a landmark directive restricting the use of the term “ORS” (Oral Rehydration Salts) in beverage branding. This decision has sparked widespread discussion across India’s food and beverage industry. Especially, as many health drink brands were leveraging “ORS” in marketing—even when their products did not meet the strict medical composition required for genuine oral rehydration solutions.
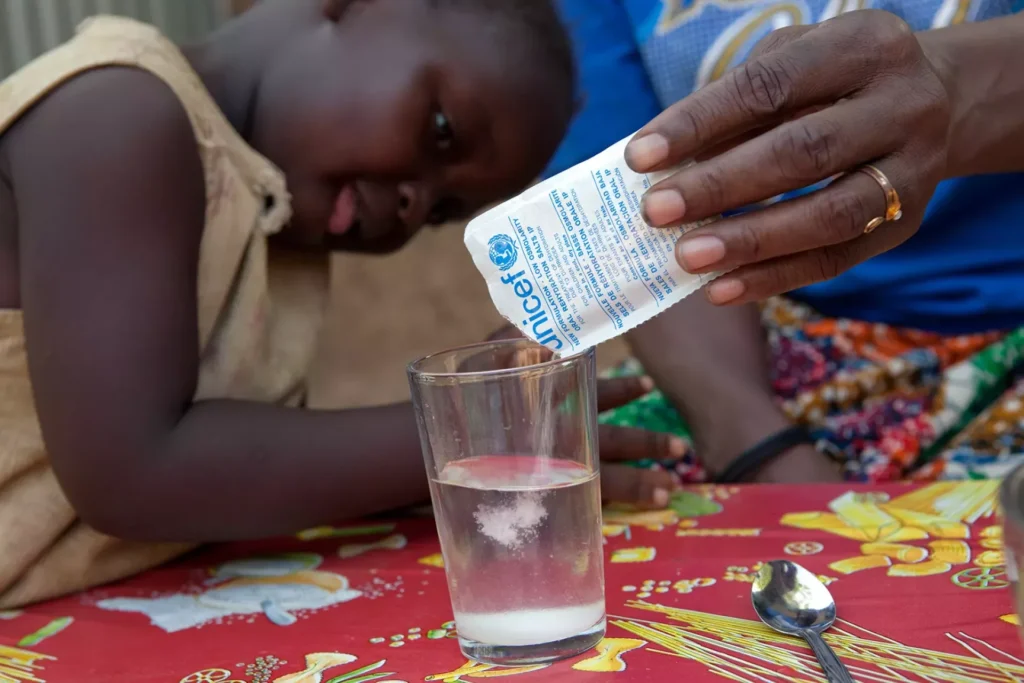
This move is more than regulatory housekeeping. It’s a clear message to the market: health claims must be backed by science, not just marketing language. The term “ORS” carries a precise medical meaning, referring to a scientifically balanced solution of electrolytes and glucose, essential for treating dehydration caused by diarrhea, vomiting, or heat-related illnesses.
Yet, flavored drinks and sports beverages have been using “ORS” misleadingly, suggesting therapeutic benefits they cannot deliver. According to the FSSAI, such labeling risks deceiving consumers, who may believe these drinks possess medicinal properties.
What Exactly Is An Authentic ORS?

An authentic Oral Rehydration Salt solution follows a standardized medical formulation recommended by the World Health Organization (WHO). It contains a precise balance of sodium chloride, potassium chloride, trisodium citrate, and glucose, enabling rapid rehydration for individuals who have lost fluids and electrolytes.
Genuine ORS is medically oriented, not designed for taste or refreshment. It is administered in hospitals, pharmacies, and healthcare centers to manage dehydration resulting from illness or extreme heat. In contrast, many beverages marketed as “ORS drinks” on supermarket shelves are high in sugar, flavored, and have inconsistent electrolyte ratios. While they may offer temporary hydration, they do not meet the therapeutic standards set by WHO or FSSAI.
The Doctor Who Fought For 8 Years
Dr. Sivaranjani Santosh, a pediatrician based in Hyderabad, waged an eight-year battle to protect the integrity of ORS and is credited with this critical reform. Alarmed by children falling ill after consuming sugary drinks labeled as “ORS,” she collected evidence, filed complaints, and ultimately petitioned the Telangana High Court to challenge misleading labeling practices.
Her persistence culminated on October 14, 2025, when the FSSAI issued its decisive directive banning misleading ORS branding. She remarked, “This is not my victory — it’s a victory for every parent, every child, and every doctor who believes that medicine must remain medicine.”
Why does the FSSAI Directive Matter?
The FSSAI’s directive reinforces consumer trust and transparency in food labeling. Only products that strictly meet the prescribed Oral Rehydration Salts composition may now use the term on packaging, advertising, or marketing. The FSSAI prohibits products with names like “Herbal ORS Drink” or “Energy ORS+” unless they meet medical standards. Manufacturers who ignore this guideline face penalties, product recalls, or license suspension under the Food Safety and Standards Act.
Startups and established companies are racing to position their products as health-boosting in India’s booming wellness beverage market. FSSAI’s message is clear: innovation is welcome, but health claims must be accurate and evidence-based.
ORS Safety and Consumer Awareness

For consumers, this is a reminder to read labels carefully. Not every “hydrating” or “energy-boosting” drink qualifies as medical-grade ORS.
Before purchasing, check for:
- FSSAI certification and batch number
- Ingredient composition (look for correct sodium, potassium, citrate, and glucose ratios)
- Absence of misleading claims like “medicated,” “therapeutic,” or “Oral Rehydration Salts-inspired” unless scientifically validated
By understanding what real ORS is—and what it isn’t—consumers can make informed choices and avoid falling prey to marketing spin.
Industry Impact: Toward Honest Branding
For beverage manufacturers, this marks a pivotal moment in functional drink marketing. Companies using “ORS” for brand appeal must now rebrand or reformulate their products to comply with the new rules.
Many brands are likely to pivot toward clearer categories, such as electrolyte beverages, hydration tonics, or energy drinks, leaving behind the misleading medical associations. Over time, this can strengthen credibility and elevate consumer confidence in the functional beverage market. Experts believe this directive will also encourage scientific formulation, responsible marketing, and transparent communication between brands and consumers.
Final Thoughts: Protecting ORS Integrity in India
The FSSAI’s crackdown on the misuse of “Oral Rehydration Salts” is more than bureaucratic enforcement—it’s a reaffirmation of public health integrity. Thus, by distinguishing between medical formulations and commercial beverages, the authority ensures that therapeutic terms remain grounded in science.
Finally, for brands, it’s a wake-up call to build trust through honesty and compliance. For consumers, it’s a nudge to look beyond the label and understand what’s truly inside the bottle.
In the end, real ORS saves lives—and that distinction is worth protecting.
Read more at The World Times.



